Page 112 - 2022 Taiwan Health and Welfare Report
P. 112
▏Section 4 Safety Management of Medical Devices and
Cosmetics
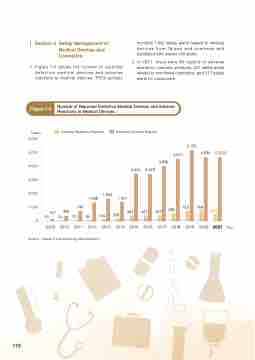
1. Figure 7-5 shows the number of reported defective medical devices and adverse reactions to medical devices. TFDA actively
monitors 1,802 safety alerts related to medical devices from Taiwan and overseas and translated and issued 143 alerts.
2. In 2021, there were 80 reports of adverse events for cosmetic products, 251 safety alerts related to monitored cosmetics, and 217 safety alerts for consumers.
Figure 7-5
Cases
6,000
5,000
4,000
3,000
2,000
Number of Reported Defective Medical Devices and Adverse Reactions to Medical Devices.
Adverse Reactions Reports
Defective Devices Reports
5,156
4,510
4,634 4,634
769
1,368 1,634 1,401 30 54 70 85 144
3,996 3,450 3,429
580
1,000 748
157 366 209 395 427 423
753
584
0
2009 2010 2011 2012 2013 2014 2015 2016 2017 2018 2019 2020 2021 Year
Source : Taiwan Food and Drug Administration
110